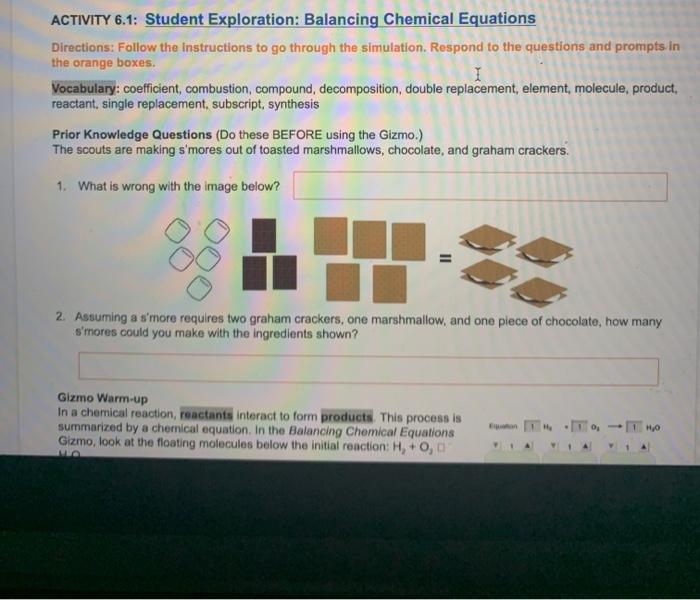
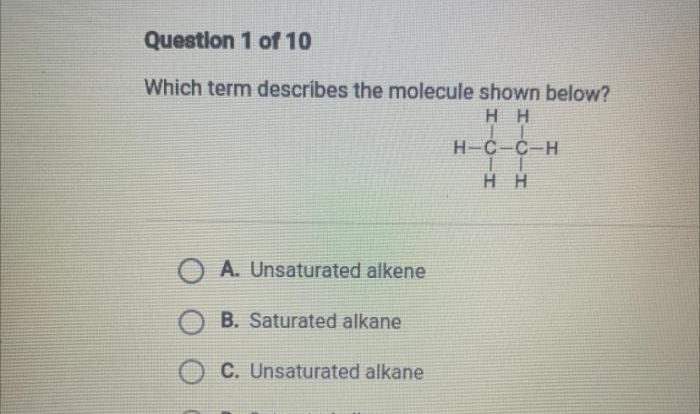
Embarking on a scientific expedition, the Student Exploration Chemical Equations Answer Key unveils the intricacies of chemical reactions, empowering students to decipher the language of chemistry. Through engaging hands-on activities and comprehensive explanations, this invaluable resource transforms the study of chemical equations into an interactive and enlightening experience.
Delving into the fundamental principles of chemical equations, this guide illuminates the significance of balancing chemical equations, ensuring the conservation of mass and providing a deeper understanding of chemical transformations. By fostering student-led exploration, the answer key empowers learners to actively engage with the subject matter, fostering a profound comprehension of chemical reactions.
Chemical Equations
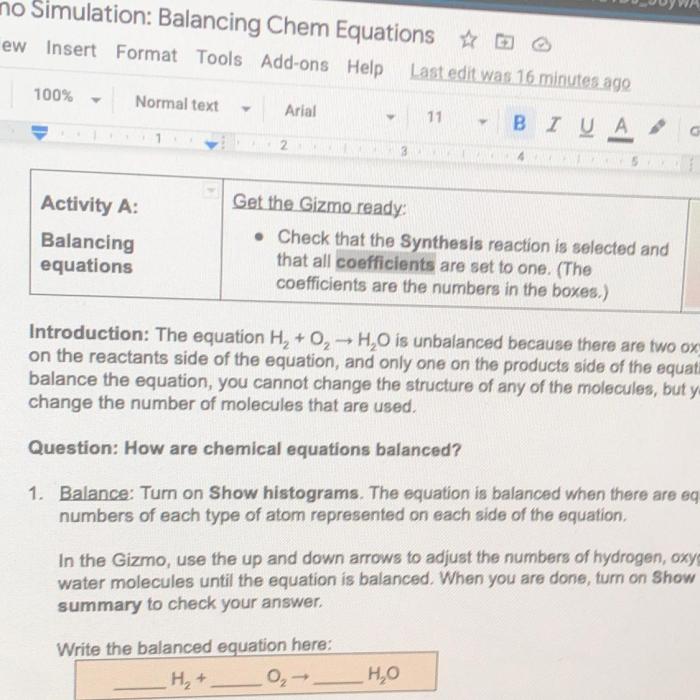
Chemical equations are symbolic representations of chemical reactions. They use chemical symbols and formulas to show the reactants, products, and the stoichiometry of the reaction. A balanced chemical equation shows the number of atoms of each element that are present on both sides of the equation, ensuring that the law of conservation of mass is upheld.
For example, the combustion of methane can be represented by the following balanced chemical equation:
CH 4+ 2O 2→ CO 2+ 2H 2O
This equation shows that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water. The coefficients in front of each chemical formula indicate the number of molecules or moles of each reactant and product involved in the reaction.
Student Exploration
Students can explore chemical equations through various methods, including:
- Hands-on activities:These activities allow students to physically manipulate molecules and observe chemical reactions firsthand. For example, students can build models of molecules using molecular modeling kits or conduct experiments to investigate the factors that affect the rate of a reaction.
- Simulations:Computer simulations provide students with a virtual environment to explore chemical reactions. These simulations can be used to visualize the movement of molecules and track the changes in concentration over time.
- Student-led exploration:By guiding students through inquiry-based learning, they can develop a deeper understanding of chemical equations. This approach encourages students to ask questions, make predictions, and design their own experiments to investigate chemical reactions.
- Balancing chemical equations:This section explains the steps involved in balancing chemical equations, including the concept of mole ratios and the use of coefficients.
- Types of chemical reactions:This section discusses the different types of chemical reactions, such as combination, decomposition, single displacement, double displacement, and combustion reactions.
- Stoichiometry:This section covers the calculations involved in stoichiometry, including determining the limiting reactant, calculating the theoretical yield, and percent yield.
- As a resource for students:The answer key can be used by students to check their understanding of chemical equations and to review the concepts covered in class.
- As a teaching tool:The answer key can be used by teachers to provide explanations and examples to students during lessons.
- As a basis for assessments:The answer key can be used to create quizzes, tests, and other assessments to evaluate student learning.
Answer Key
The answer key provides detailed explanations and examples to support the answers to common questions related to chemical equations. It is organized into the following categories:
Classroom Applications, Student exploration chemical equations answer key
The answer key can be used effectively in the classroom in the following ways:
FAQ: Student Exploration Chemical Equations Answer Key
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side equals the number of atoms of that element on the products’ side, upholding the law of conservation of mass.
How can hands-on activities enhance the understanding of chemical equations?
Hands-on activities provide a tangible and interactive approach to learning, allowing students to visualize and manipulate chemical reactions, deepening their comprehension of the concepts.
Why is student-led exploration beneficial in learning about chemical equations?
Student-led exploration fosters critical thinking, problem-solving skills, and a deeper understanding of the underlying principles governing chemical reactions.